Saad Yalouz, Vincent Robert and coworkers published a new paper, “Modulated Ligand–Ligand Exchange Coupling and Elusive Spinmerism in a Bis(verdazyl)iron(II) Complex” in Inorganic Chemistry : https://doi.org/10.1021/acs.inorgchem.5c01378
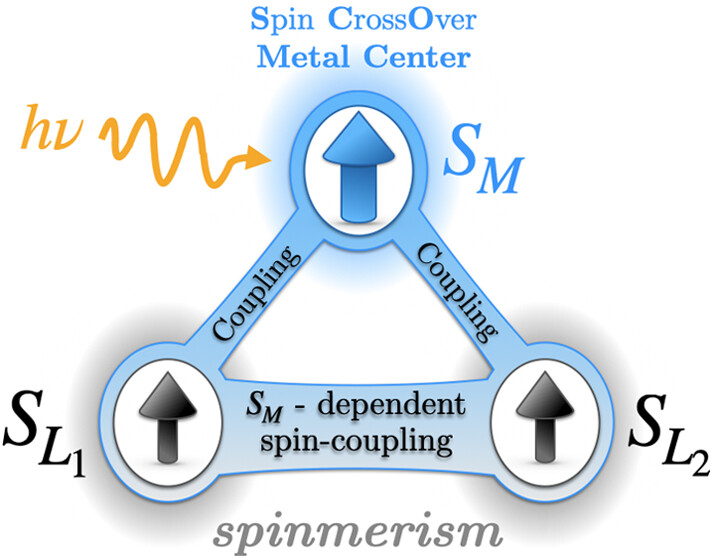
1,5-Dimethyl-3-(2,2′-bipyridine-6-yl)-6-oxoverda-zyl (bipyvd) was used as a tridentate radical ligand toward iron(II) perchlorate to yield bis(1,5-dimethyl-3-(2,2′-bipyridine-6-yl)-6-oxoverdazyl)iron(II) perchlorate, [Fe(bipyvd)2] (ClO4)2. The single-crystal X-ray Fe–N distances are compatible with a low spin (SFe = 0) iron(II) center surrounded by two bipyvd radical ligands. A similar conclusion is reached from difference dedicated configuration interaction (DDCI) wave function calculations, which support the coupling of radicals through a closed-shell metal ion bridge. The singlet–triplet splitting is ferromagnetic, with a calculated exchange coupling constant JLLSFe = 0 = +120 cm–1. Higher in energy, the calculated spin states split into two subsets successively characterized by pure local spin states SFe = 2 and SFe = 1. In contrast, the coordination sphere exhibits either a pure local spin state or a superposition of SL = 1 and SL = 0 local spin states, a phenomenon previously coined into spinmerism. A marked modification of the ligand–ligand ferromagnetic coupling is evaluated, with JLLSFe = 2 = +89 cm–1 and JLLSFe = 1 = +140 cm–1, whereas the metal–ligand interactions remain almost constant at ca. +290 cm–1. The control of the magnetic interactions between organic radicals through a spin-crossover ion revisits the traditional picture dominated by the metal ions and stresses the manifestation of spin entanglement between the coordination sphere and the metal center environment.